- energy is quantized
- particles display wave-like behavior
Energy Quantization
- evident through black-body radiation experiment by Plancke
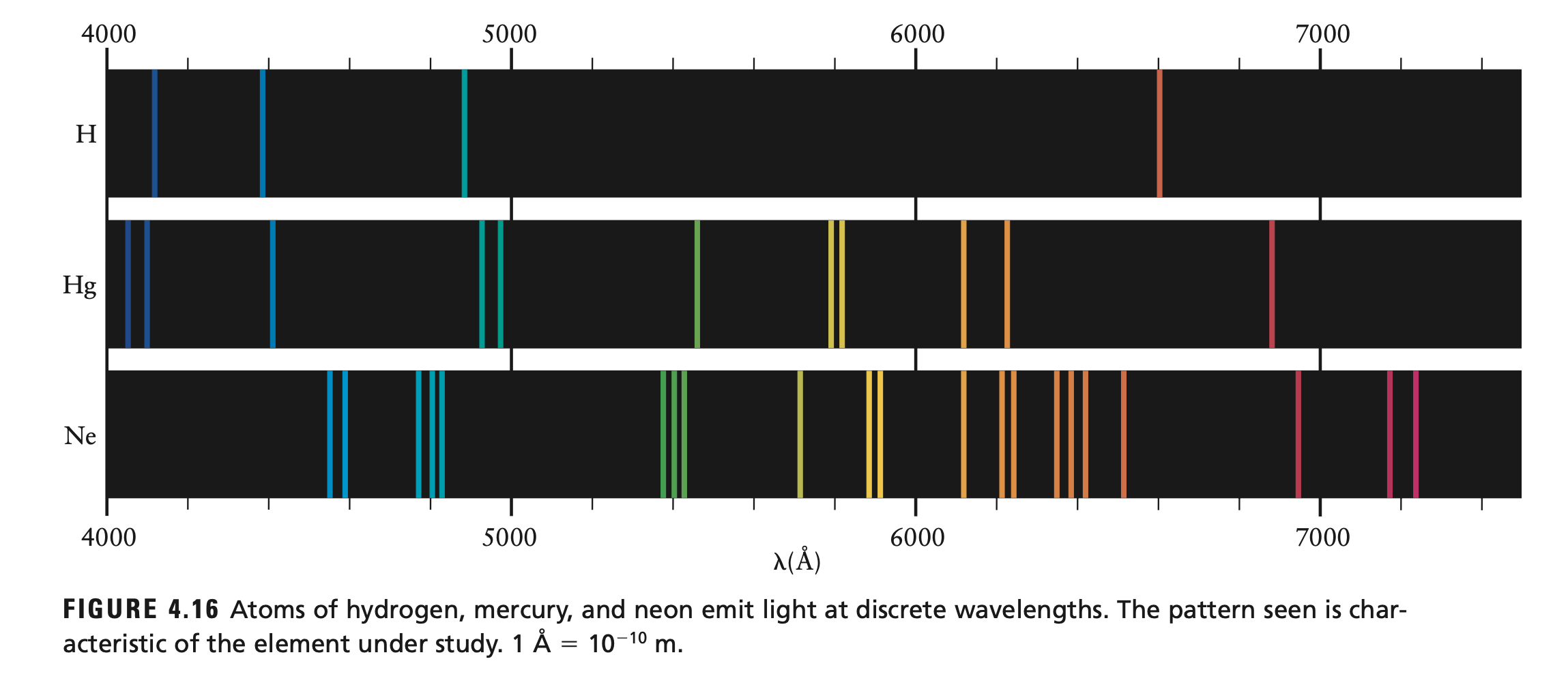
- evident through atomic emission spectrum
- Franck-Hertz experiment showed the atoms have energy levels because electrons colliding with gaseous atoms only gain energy when they have a certain energy at a threshold voltage
Bohr Model
- connects continuous energy to quantized energy of electron seen above
- model of hydrogen atom with discrete energy states
- frequency of light absorbed is related to change in energy state
- based on Rutherford’s model: electron orbits around nucleus
- is effective nuclear charge
- can relate force to magnitude of force with velocity using centripetal motion
- impossible in classical physics
- electron is accelerating implies emits EM radiation
- should eventually collide with nucleus
- Bohr avoids above conflict by assuming electron can only occupy discrete orbits
- characterized by radius , energy
- angular momentum is also quantized in multiples of , is Plancke’s constant
- can solve for , plug into force equation, get
- is Bohr’s radius, allows us to get clean formula
- can substitute and to get
- expressed in energy unit rydberg
Ionization Energy
- consistent with IE of hydrogen atom
- ionization is equivalent to Bohr model transition from
- multiply above value by Avogadro’s number to get yields the correct value for IE
Atomic Spectra
- can also be used to explain colors in atomic spectra
- since , we get lines of light of certain colors (frequencies)
Wave-Particle Duality
- Bohr’s model modeled energy quantization but didn’t explain origin
- need concept of wave-particle duality
- particles can behave like waves and vice versa
Photoelectric Effect
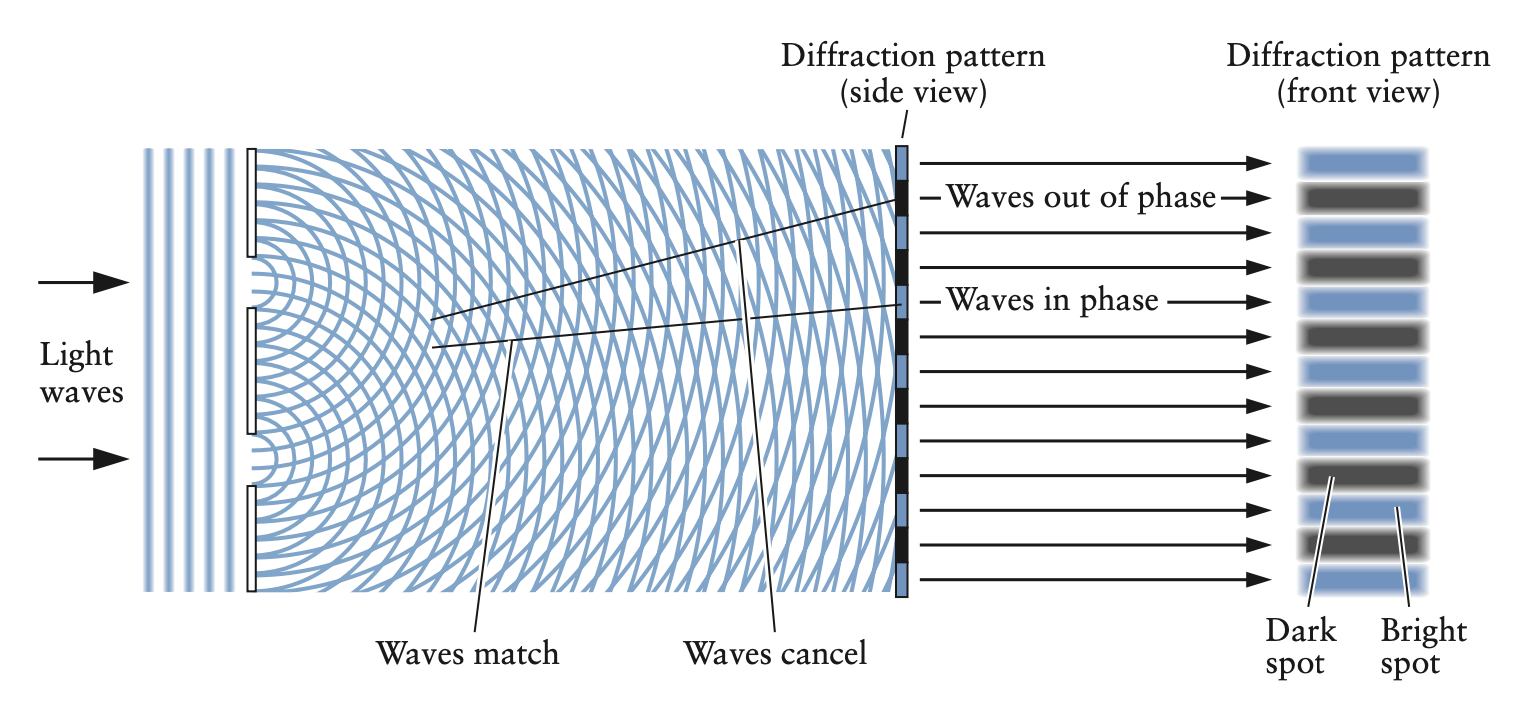
- light behaves like waves through constructive and destructive interference in experiments
- light waves also behave like particles (photons)
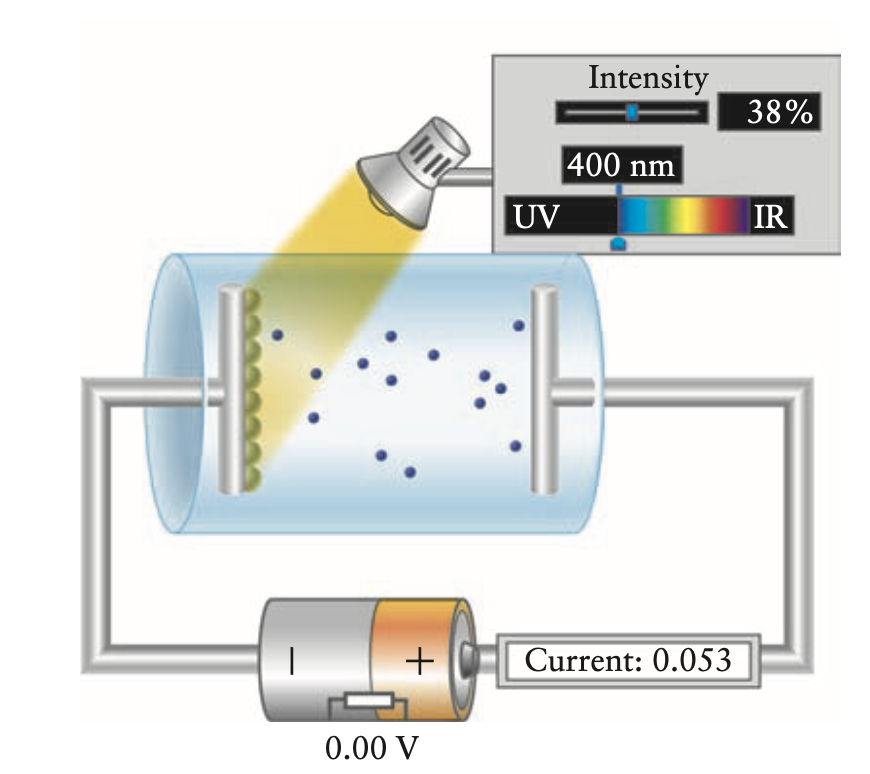
- light shining on surface of metal can eject electrons (photoelectrons) and create current
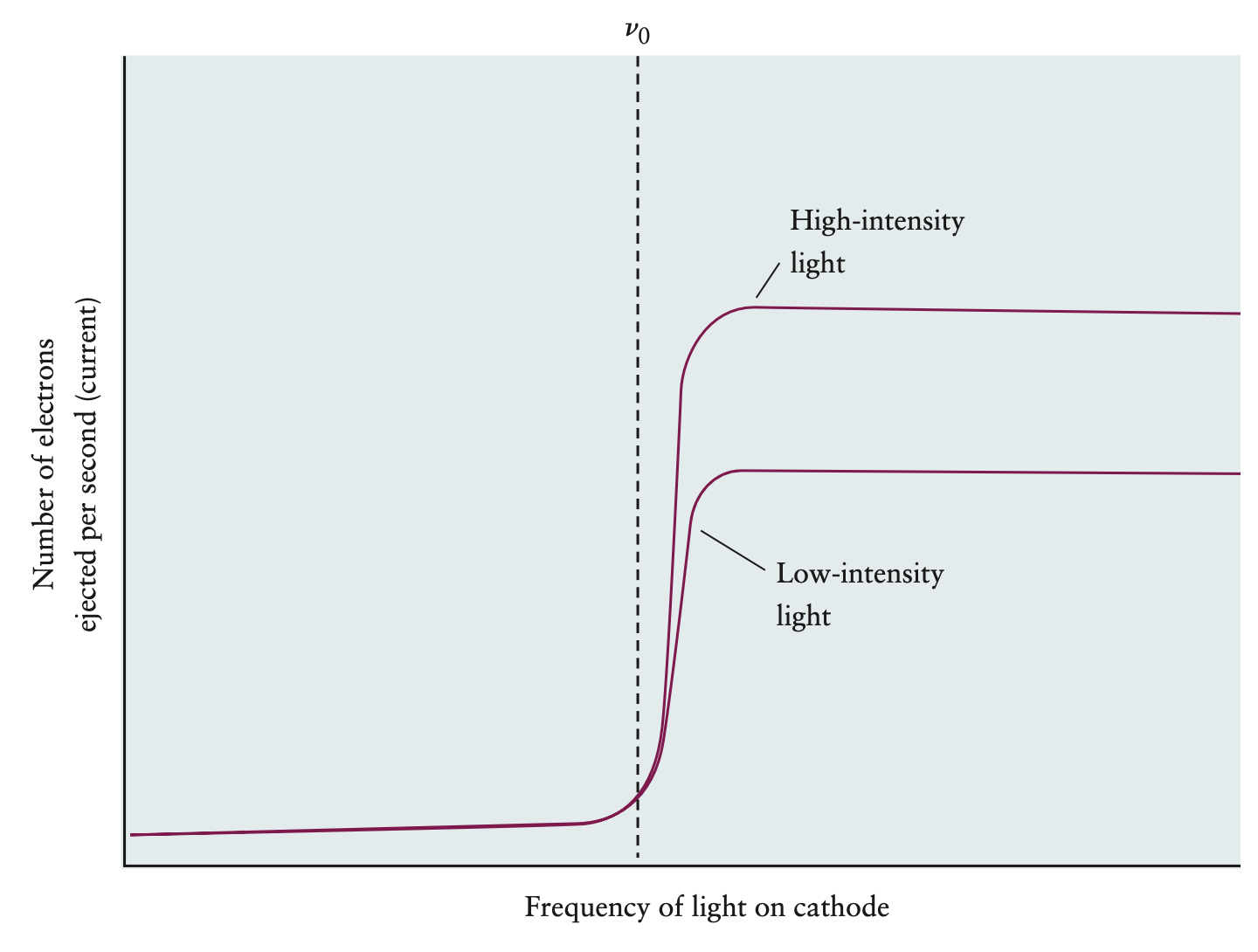
- electrons are only ejected after a certain threshold frequency of light
- intensity doesn’t cut it
- figure below relates intensity, frequency, current
- voltage applied by battery in experiment diagram can be used to measure the energy required by the light
- electron has less maximum kinetic energy energy than means it won’t make it across voltage, thus no current
- concept of photon explains connection between threshold frequency and max kinetic energy
- photon is quanta of energy of light, like a particle
- metal absorbs photon, photoelectron gains energy to escape nucleus
- photoelectron loses energy from collisions with other atoms and in escaping the surface
- conservation of energy:
- electrons with come from surface, where
- thus,
- is a constant characteristic of metal
- key point of Einstein’s photon is that photon does all or nothing
De Broglie Waves
- EM radiation behaves like traveling waves
- : amplitude
- : wavelength
- : frequency
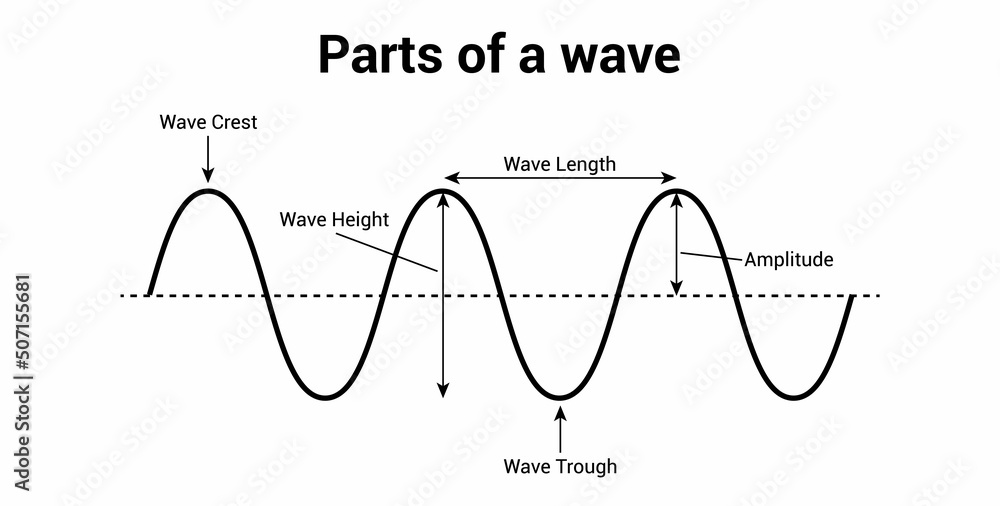
- there are also standing waves
- example: guitar string is fixed at both ends (boundary conditions), thus only certain oscillations are possible
- condition for allowed wavelengths is
- every value of corresponds to a harmonic
- higher harmonic has more nodes, aka points in wave where
- De Broglie standing waves perfectly describe quantization
- quantum number relates to harmonics
- electrons behave like circular standing waves

- matches the relationship for angular momentum described by Bohr
- De Broglie used theory of relativity to extend this to photons too
- generalization: any particle moving with linear momentum has wavelike properties and
- De Broglie waves imply Heisenberg Uncertainty Principle
- cannot know position of momentum at any one time
- wave-like properties imply electrons can be modeled by wave function, which led to
Schrodinger’s Equation
- is the electronic wavefunction
- depends on , aka position of electron and time
Origin
Consider a particle moving freely along the x-axis with momentum . It must have wavelength . Two wave functions that work are
The second derivative of either function is
Replacing with from de Broglie’s relation, we get
where is the kinetic energy of the particle. Including external forces from the walls around the particle or the presence of fixed charges and using to represent the total energy, we get
Letting the Hamiltonian operator denote the energy of the wave function, we get
Interpreting Energy
- potential doesn’t vary with time
- Schrodinger’s equation can only be solved for specific values of
- thus, Schrodinger’s equation energy quantization
- time independent wave function state = stationary state
- every stationary state has different energies and different wave functions
- lowest energy = ground state, other energy = excited state
Interpreting Wave Function
The classical electromagnetism view of light states
If light is photons, the intensity is the probability of finding a photon. This analogy is the probability density of a particle is the probability density function:
is the probability that a particle will be found in a region of volume centered at .
Probability density is normalized since sum of all probabilities is 1:
Normalization also implies that
- are continuous
- is bounded, or as , .
Solving the Equation
- can simulate solving the equation with a Particle in a Box Model simulation
- not complete solution, only for one electron system
- solution for energy demonstrates energy quantization
- can’t actually solve equation for real case scenarios
Relationship to Atomic Orbitals
- solution of Schrodinger’s Equation for one-electron atom when replacing potential energy with Coulomb potential quantizes 3 values, known as quantum numbers
Molecular Orbitals
- Quantum mechanics can be used to explain Molecular Orbitals