Quantum Picture of Chemical Bond
As 2 atoms approach each other,
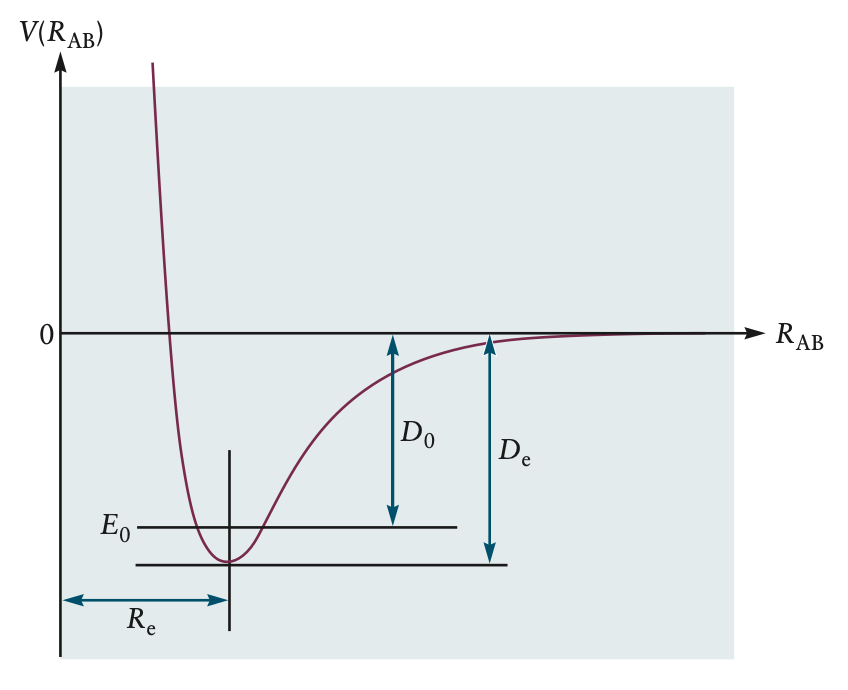
- effective potential energy curve is caused by repulsive forces between nuclei, attractive forces between nuclei and electrons, and repulsive forces between electrons
- lower energy = more stable understanding potential energy is key to understanding molecule
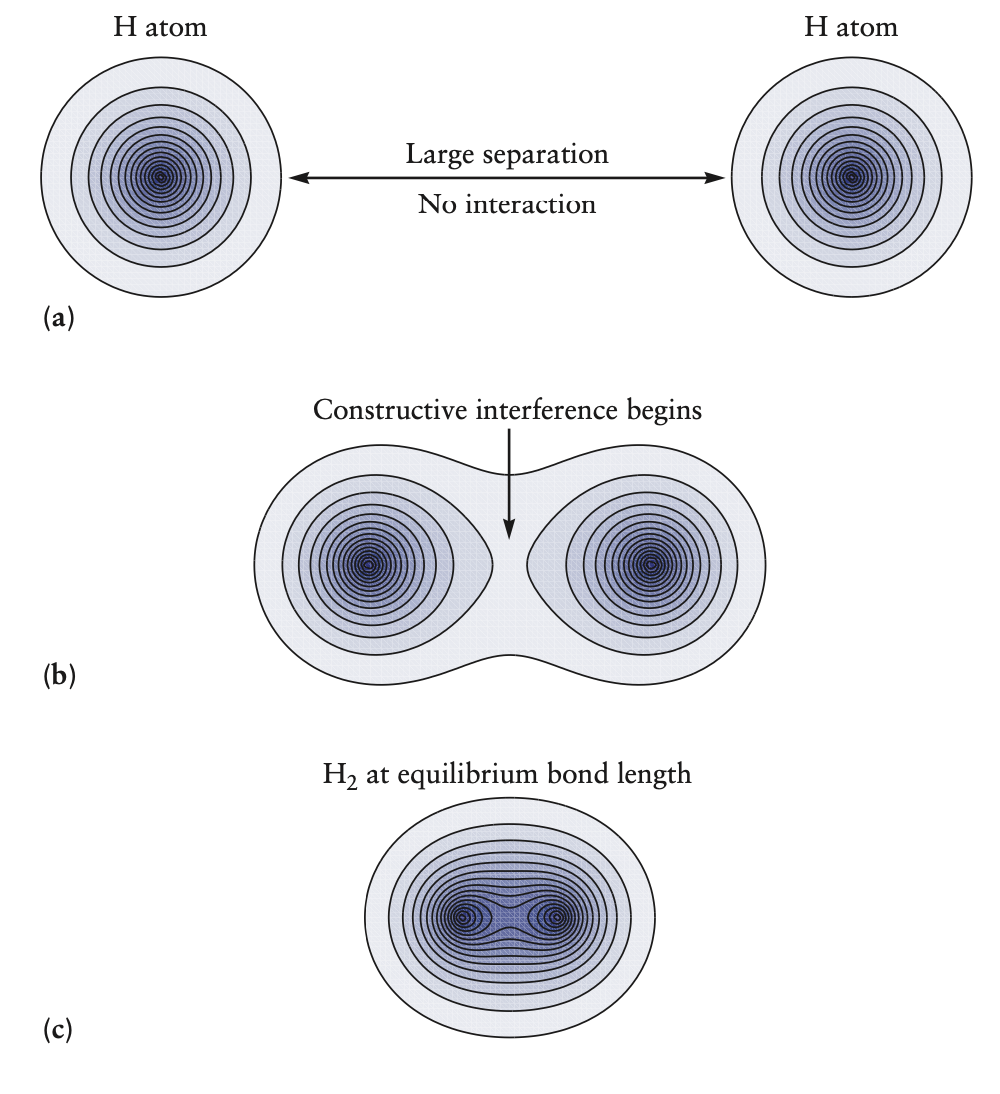
- above picture of two hydrogen atoms can also be interpreted as the merging of wave functions of electrons
- can mathematically calculate effective potential energy using
Born-Oppenheimer Approximation
- electrons move much faster than nuclei
- assume nuclei are fixed in space when solving Schrodinger’s equation
- 2: integer that tracks relative energies for each symetry type
- : descirbes orientation around internuclear axis
- : cylindrical symmetry
- : has nodal plane that contains internuclear axis
- : describes inversion symmetry
- : ungerade, : gerade
- *: node on internuclear axis