- holds atoms together
- change the properties
- care about nature of bond and resulting shapes
- example: graphene vs polyethylene
- insert hexagon vs chain
Ionic Bond
- complete transfer of one or more electrons from one atom to another
- has dipoles
- formed between 2 atoms of differing electronegativity ()
- example: . electron goes from Na to. Cl
- IE of Na is 495.8
- IE of Cl is 1251.2
- Cl is 3p orbital but less energy than Na because increased number of protons, but not increased principle quantum number (distance from nucleus)
- atoms that form ions w/ noble gas configurations are very stable
- NaCl is example once again
- Na -> Ne, Cl -> Ar
- ,
- 8 electorns in valence shell
- octet rule: atoms with 8 electrons around them are stable
- general rule
- Group 1 likes to lose electron ->
- Group 2 likes to lose 2 electrons ->
- Group 3 likes to lose 3 electrons ->
- Group 6 likes to gain 2 electrons ->
- Group 7 likes to gain electron ->
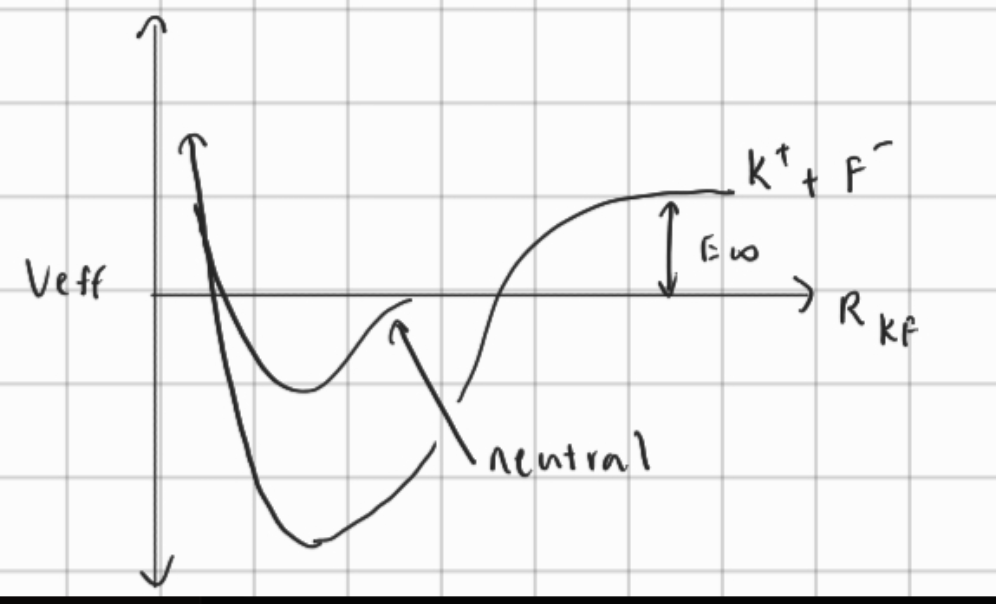
To calculate the change of energy when K bonds with F,
Basically, the potential energy for a distance of between the two ions in terms of Joules per ion pair is the above. To get it in , we need to multiply by Avogadro’s number and divide by 1000.
Covalent Bonds
- electrons are shared evenly
Polar-Covalent Bonds
- gray area between ionic and covalent
- somewhat closer to one atom than another
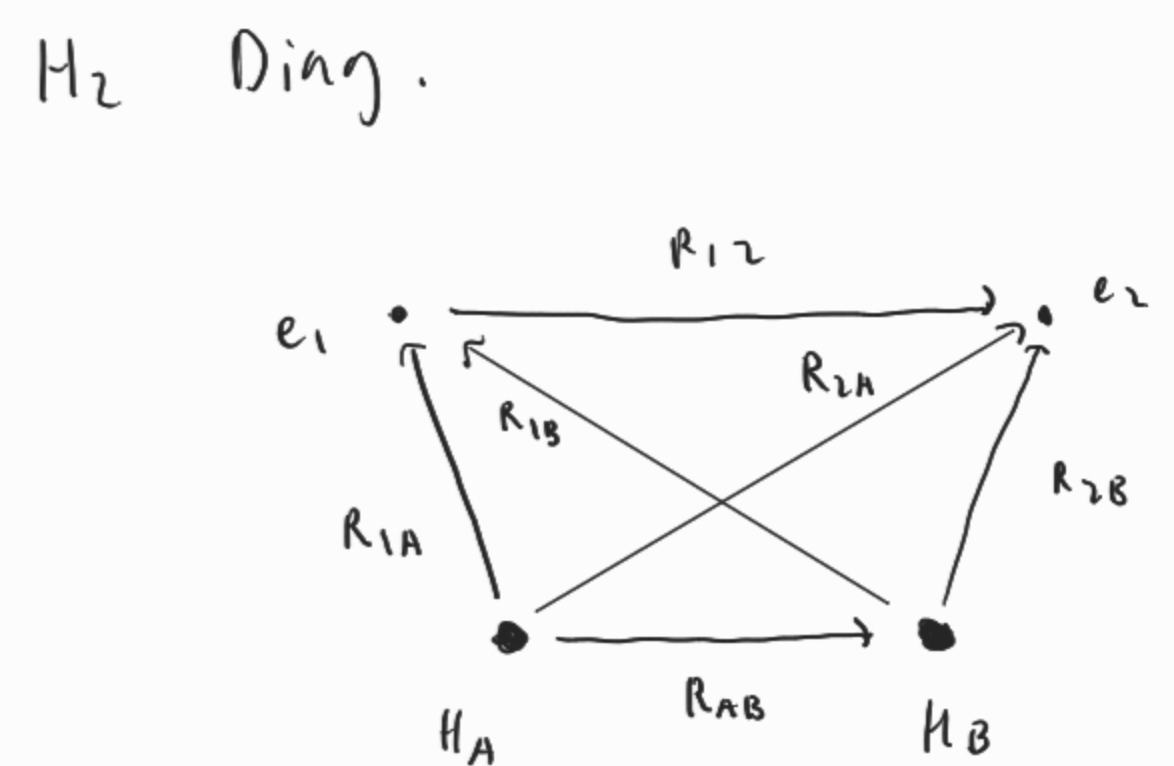
Let’s consider the simple molecule H2
- forms of energy
- potential: energy due to position or composition
- kinetic: motion
Energy
Let’s consider the simple molecule .
Potential Energy
- energy due to composition or position
- is attractive
- are repulsive
Total Energy
Morse Potential
Morse potential curve describes the potential energy in a diatomic bond as a function of the radius. The basic version looks like the diagram below.
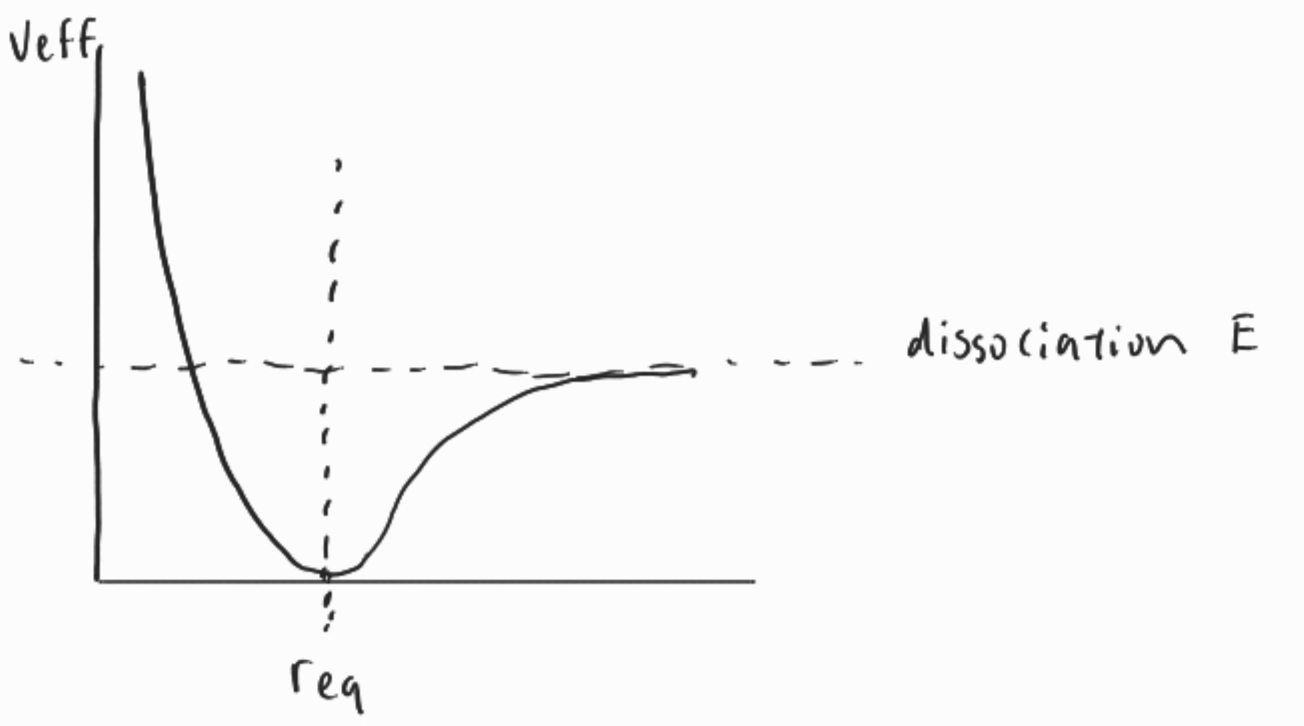
When the , the repulsion force between the electrons of one atom and another and the repulsion of the nuclei of each atom generate high potential energy. Then, as the attractive force between the electrons of one atom and the nucleus of the other is what increases potential energy.
Below is a diagram for an ionic bond.
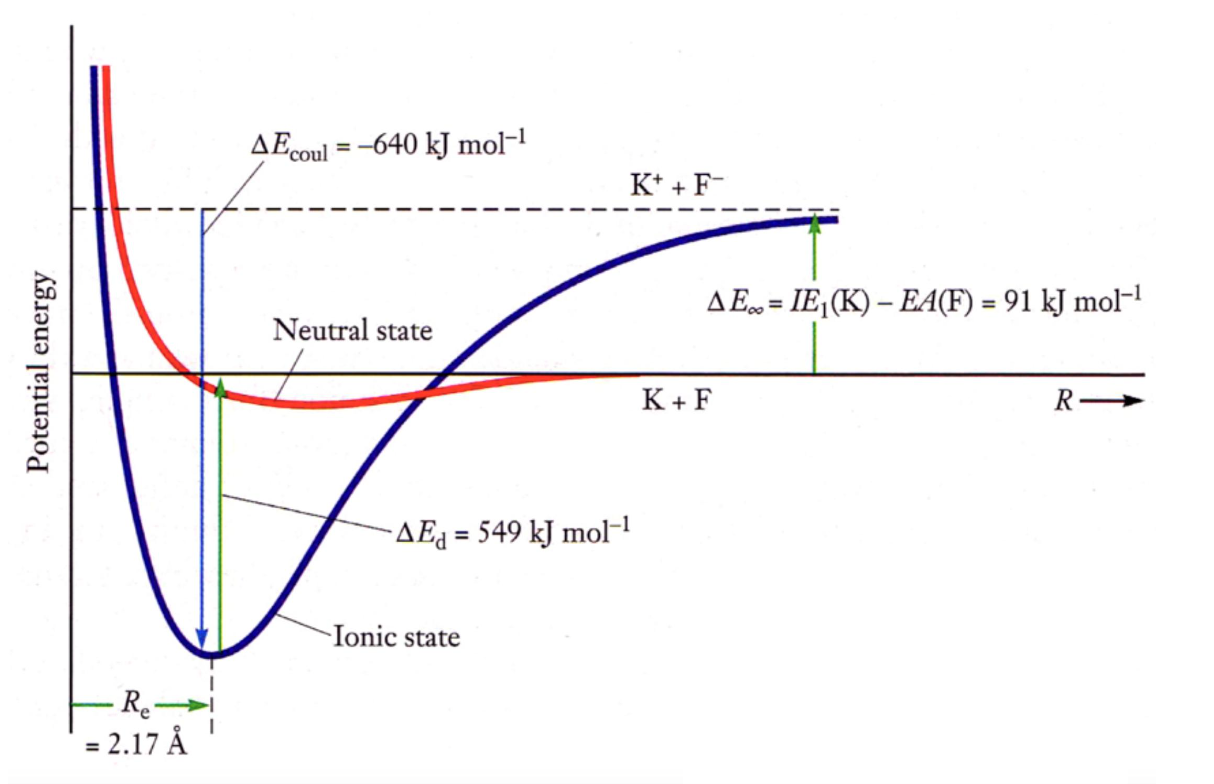
- strong ionic bond
- the potential energy is less at since the bond is super stable
- is shorter because stronger bond
- potential energy is larger because of energy required to ionize both ions in the bond
Bond Descriptors
Bond Length
- distance between 2 atoms
- shorter -> stronger
Energy
- larger = more stable
- reproducible from molecule to molecule
- C-O is
- O-H
Order
- number of shared electrons between 2 pairs of atoms
- more electrons = stronger = shorter distance
Polarity
- absolute value of the difference in electronegativity between 2 bonded atoms
- large difference > 2 => ionic
- small < 0.4 => covalent
- in between 0.4 -> 2 => polar covalent
- :
-
- fraction of charge on each atom
- is total charge
- is 1 and 0 for ionic bond
- measurable
- is dipole moment in debye
- is bond length in
- for HF, ionic
Oxidation Number/States
- oxidation numbers:
fictitiouscharges on each atom in molecule- good for you, gives you cancer
- powers phones
Rules
- number of atoms in neutral molecular must add to charge of ion (0 if neutral)
- alkali-metal atoms = +1, alkaline earth atoms = +2 b/c noble gas
- above all (unless ) and halogens = because noble gas (unless other halides or )
- unless alkali metal then
- unless with (rule 3)
Bonding in Different Families
Different families of elements bond differently.
- Bonding in organic molecules
- Bonding in Transition Metals