Lewis Dot
- predicts structural formula -> sequence of atoms bonded
- diagrams with like formal charges on adjacent atoms -> unstable
- opposite formal charges on adjacent atoms are good
- draw atom with valence
- octet rule: main group surrounded by
- 2 in s orbital, 6 in p orbital
- exception is , (surrounded by 2)
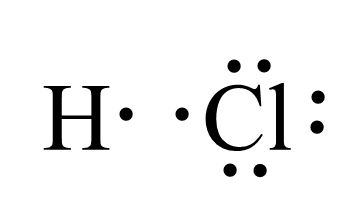
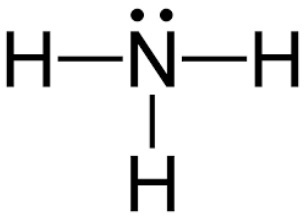
- assign formal charge
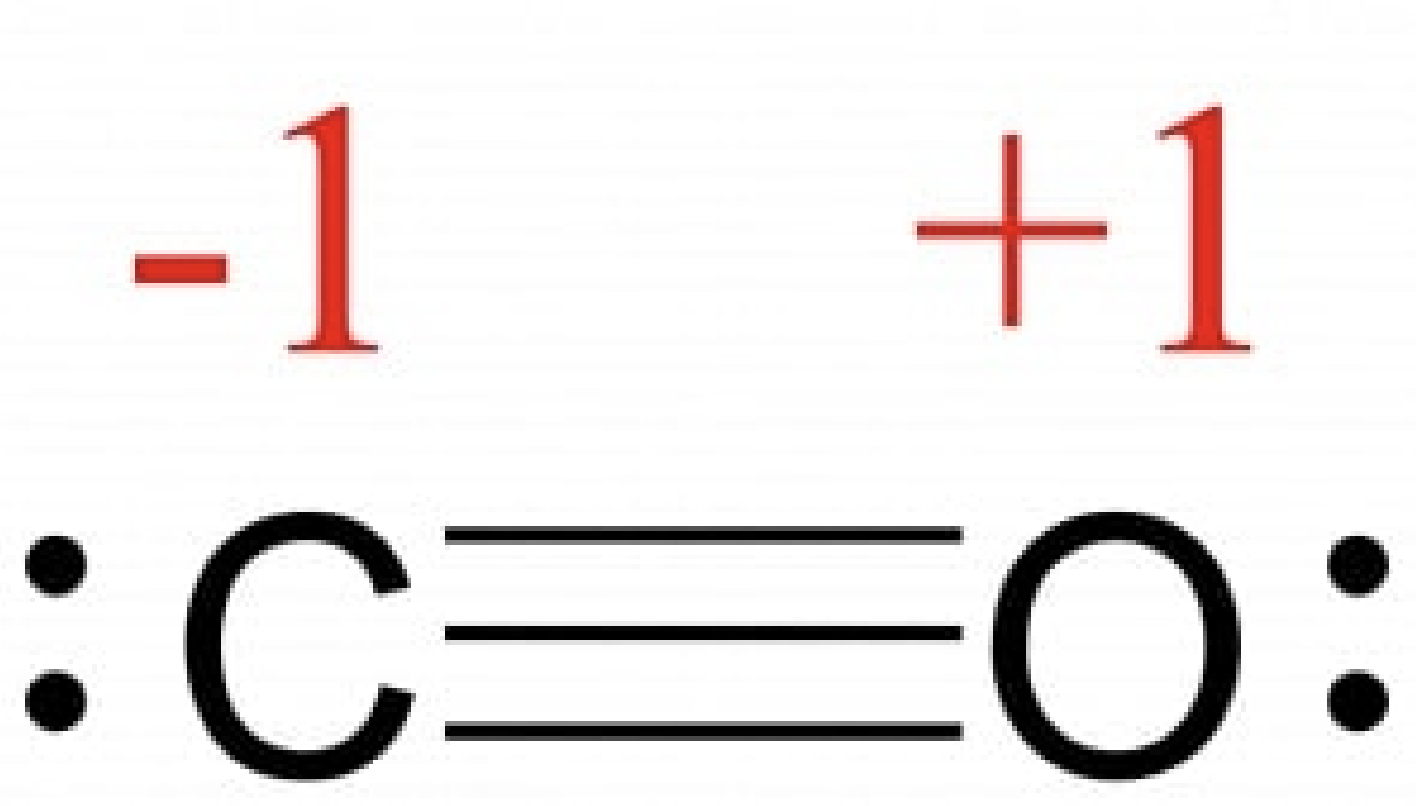
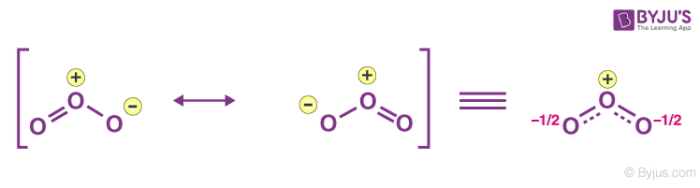
Exceptions
Ozone
- unstable
- rekt by CFC
- bond length not predicted by lewis dot
- resonance hybrid
- both bonds are 1.5 order
Other
- odd molecules (NO)
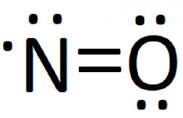
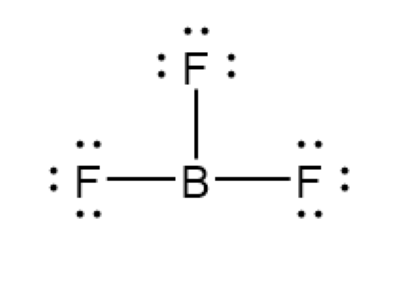
- octet-deficient molecules ()
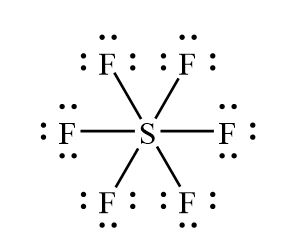
- valence shell expansion (3rd period and down)
Valence Shell Electron-Pair Repulsion Theory (VSEPR)
- predicts shape of molecule based on Lewis Dot structure
- electron pairs repel
- 3 aspects that effect reactivity
- bond length
- size/shape
- bond angles
- dihedral angle
- geometry based off of nuclear coordinates
- steric number (SN): number of atoms bonded to central atom and lone pair
| Molecule | Steric No | Predicted Geometry | Example |
|---|---|---|---|
| 2 | Linear 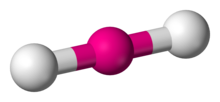 | ||
| 3 | Trigonal Planar 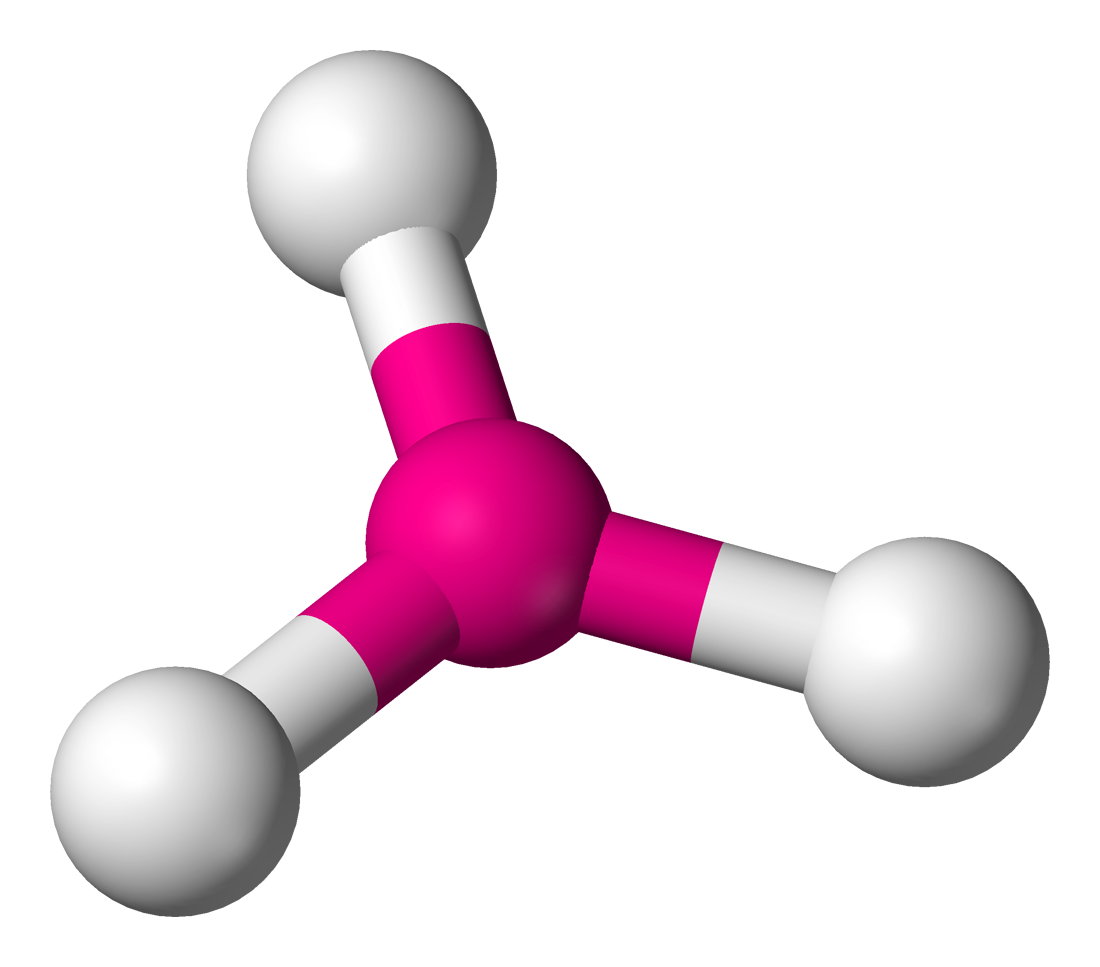 | ||
| 4 | Tetrahedral 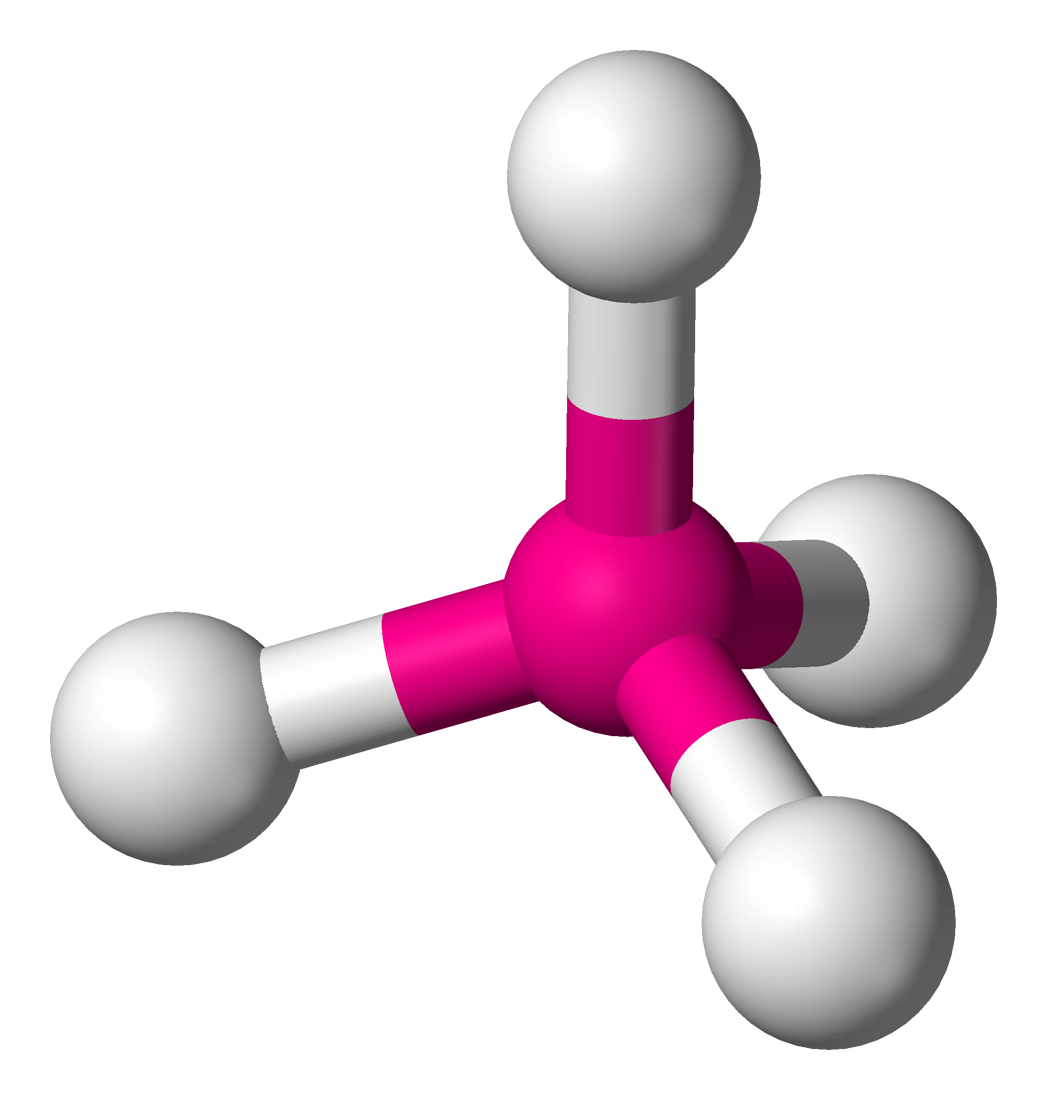 | ||
| 5 | Trigonal Bipyramidal  | ||
| 6 | Octahedra  |
- lone pairs occupy more space than bonding pairs
- dissimilar atoms cause different repulsions
- attracts away from reduces repuulsion
- electropositive repels more than electronegative atoms
Dipole moment
- unequal distribution of , vector
- good example:
- bad example:
- nonpolar