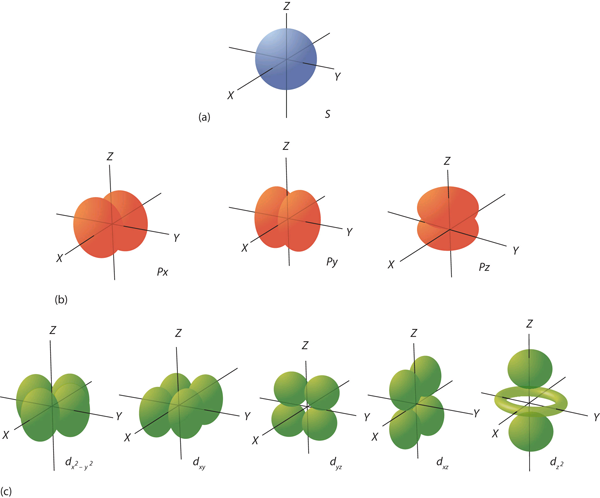
- forms cloud around nucleus
- live in orbitals around nucleus
- orbitals have size, shape, energy
- orbitals are associated with quantum numbers
Quantum Numbers
- solution of Schrodinger’s Equation for one-electron atom when replacing potential energy with Coulomb potential quantizes 3 quantum numbers :
- electrons with quantum numbers (aka every Hartree orbital) are associated with a wave function , where is the probability of finding the electron at position .
4 Quantum Numbers
Principal Quantum Number ()
- indexes energy level
- comes from quantization of energy
- energy of electron
- (distance from nucleus)
Angular Momentum Quantum Number ()
- quantization of (angular momentum squared)
- related to shape
Magnetic Quantum Number ()
- quantization of angular momentum
- describes how energy of atom shifts in external magnetic field
Electron Spin Quantum Number ()
- orientation of the magnetic moment of the electron is quantized according to Stern-Gerlach experiment
- 2 electrons live in orbital described by first 3 quantum numbers
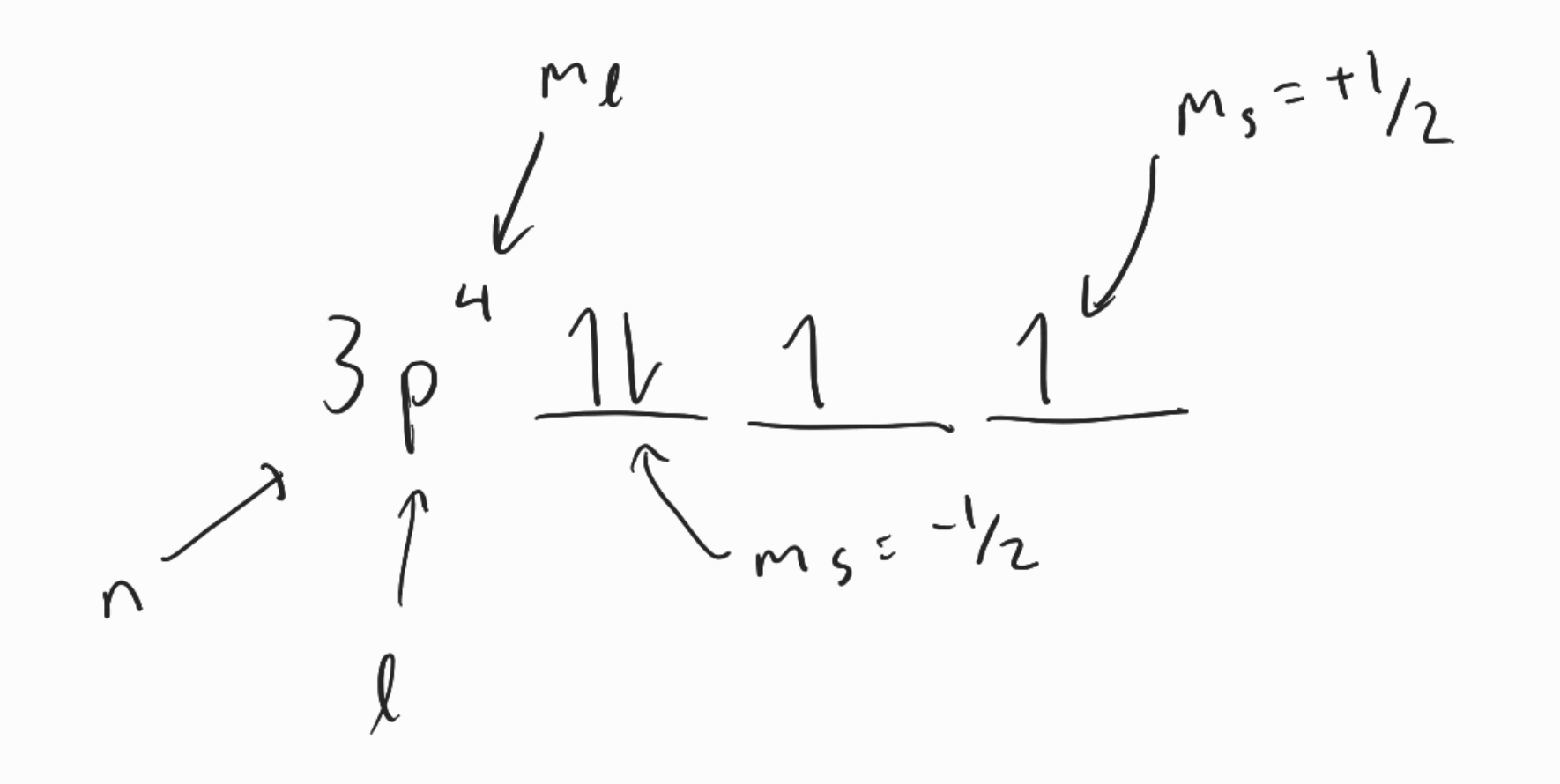
Rules
Basically, electrons ALWAYS go for LOWER ENERGY. This summarizes all of chemistry.
Aufbau Principle
- describes ground state electron configuration of atom
- ground state: lowest energy state of atom
- built up by arranging the Hartree atomic orbitals in order of increasing energy
- electrons always fill lower energy orbitals before higher energy orbitals
- Periodic Table spdf categorization is based on this
- start with on left, end with on right
- Periodic Table spdf categorization is based on this
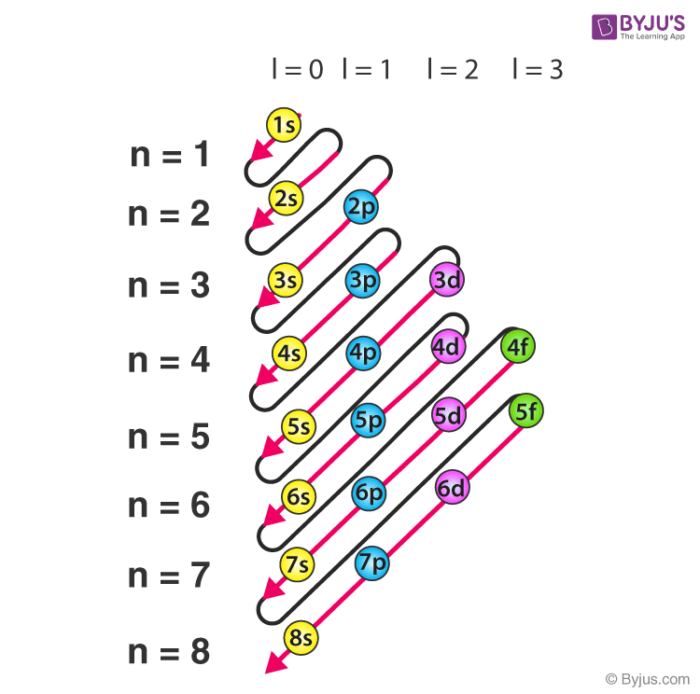
- exceptions starting in period 4
- transition metals
- energy of and subshells are very similar
-
-
- when putting >3 electrons into 3d orbitals, there is electrostatic repulsion energy ()
- because localized vs diffused
- half filled + fully filled shells -> lower energy -> electrons sometimes go to to fill last spot for fully filled/half filled subshell
Pauli’s Exclusion Principle
- no 2 atoms can have same 4 qn
Hund’s Rules
- when electrons are added to Hartree orbitals of equal energy, all orbitals will be singly occupied before any is doubly occupied
- lowest electron config has parallel spins
Electron Configurations
- Example configurations:
- H:
- He:
- Li:
- B:
- C:
Ionization Energy
- energy required to remove electron from orbitals to vacuum (0 energy)
- farther away from nucleus -> shielding lower effective nuclear charge -> higher energy -> lower ionization energy
- higher ionization energy -> more stable
- measured by photoelectron spectroscopy
- shots photon at electron with certain energy
- produces graph with peaks in energy that represents atomic subshells
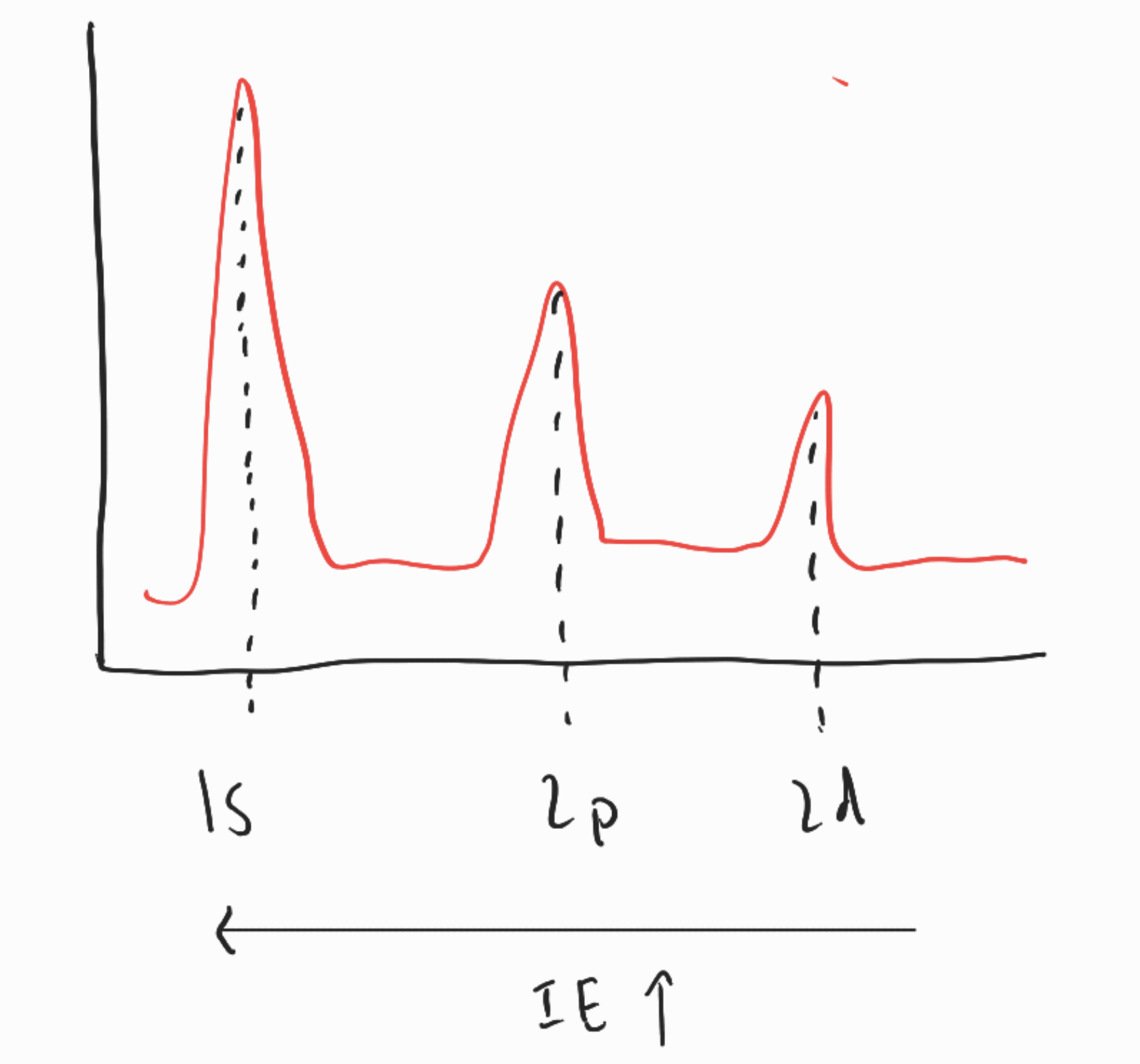
- energy level differences valence vs core electrons
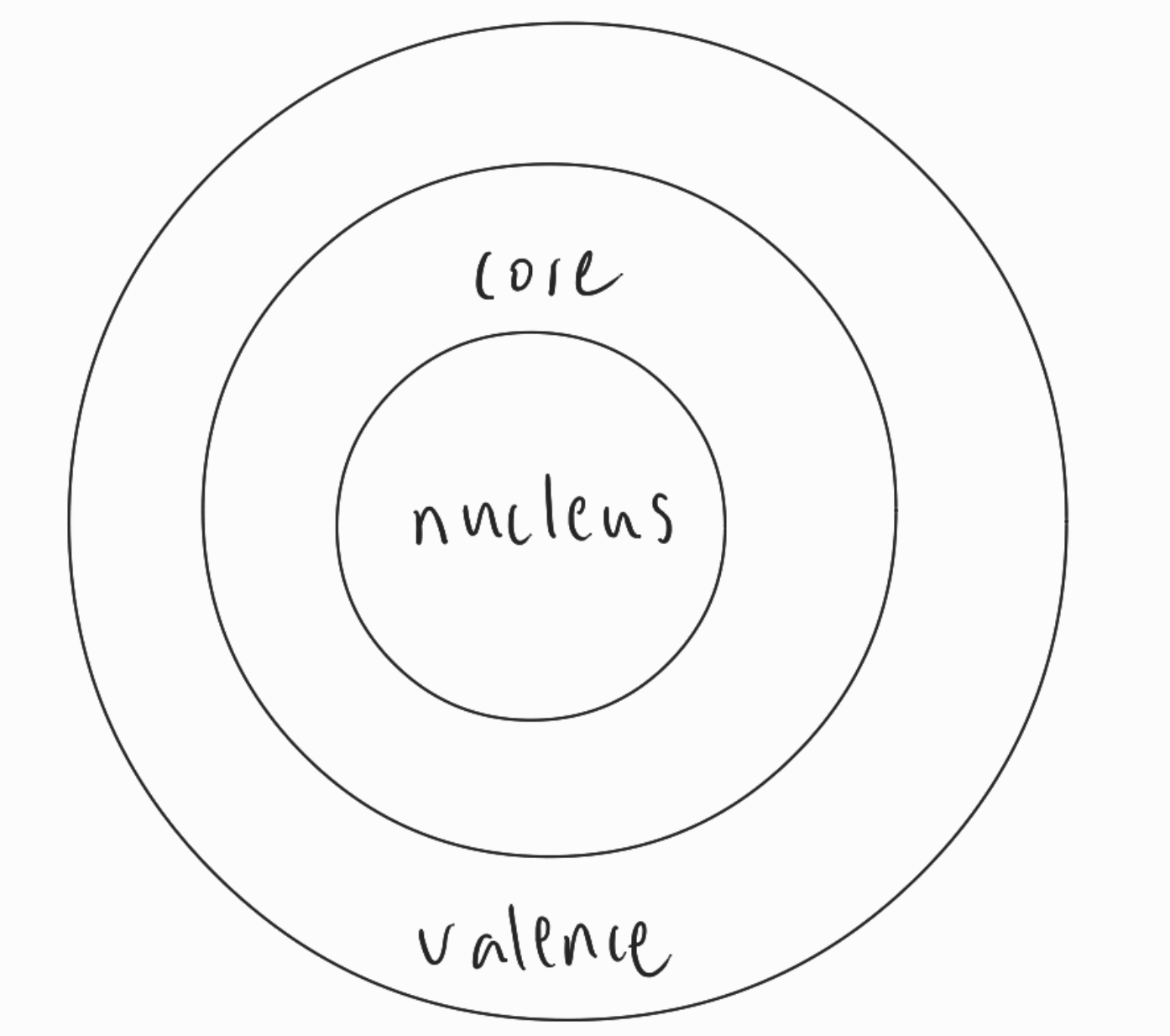
- valence electrons
- outermost shells
- participate in bonding
- core electrons
- all the others
- cause shielding
- (Ry) rydberg energy: J
- boundary between valence and core electrons
- is valence electron if is valence electorn and vice versa
Electron Affinity
- energy required to detach electron from anion to yield neutral atom
- energy released when atom gains an electron
Electronegativity
- tendency of an atom to attract share
- bonds
- relative
- atomic radius 1/EN
- nuclear charge EN
- shielding 1/EN
- example: Cl and Na
- electron from Na will go to Cl because Cl more stable
- causes dipoles in ionic bonds
There are two scales:
Mulliken
is a proportionality constant.
Pauling
- related to bond dissocation
- A-A
- perfectly covalent
- = bond dissociation E
- B-B
- = bond dissociation E
- consider A-B
- covalent character of A-B =
- ionic character = (excess bond energy)
- define in terms of
- EN diff > 2 = ionic bond
- EN diff = 0 = covalent bond