Chemistry is about
- understanding the link between atomic structure, bonding, and properties.
- rxn -> structures and electrons change
Two Fundamental Building Blocks
- matter
- conservation of matter: matter cannot be created or destroyed
- energy
- conservation of energy: energy cannot be created or detroyed
- can be converted
Dalton’s Atomic Theory of Matter
- Matter consists of indivisible atom
- All atoms of given element are identical in mass + properties
- Atoms are indestructible and retain IDs during reaction
- Atoms can combine to make compounds
Subparticles
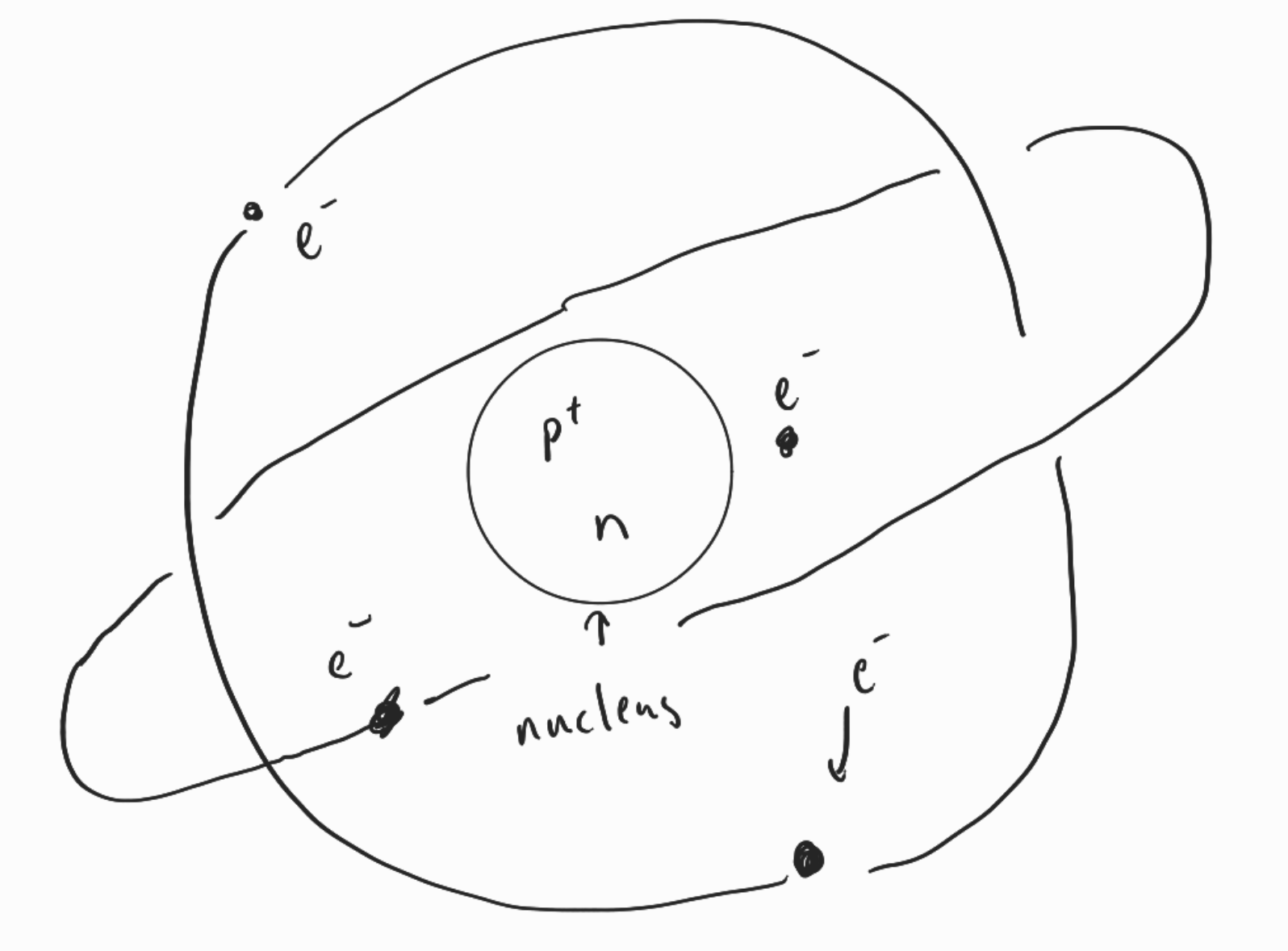
Electrons in Atoms
- forms cloud around nucleus in orbitals
Nucleus
- proton
- neutron
- mass number: sum of number of and number of
- defines isotope
- atomic number: number of
- defines element
Relation to mass
- need to correlate atoms to mass
- use Avogadro’s Number
- 1 mol = molecules, atoms, entities
- atomic mass units (amu)
- unit of mass for atomic and molecular weights
- of atom mass
- molar mass (atomic mass/atomic weight/molecular weight)
- each atom has molar mass
-
- To find molecules in ,
- molecules